High-Performance Liquid Chromatography and Supercritical Fluid Chromatography
Part 4 of our "What is Chromatography Series"
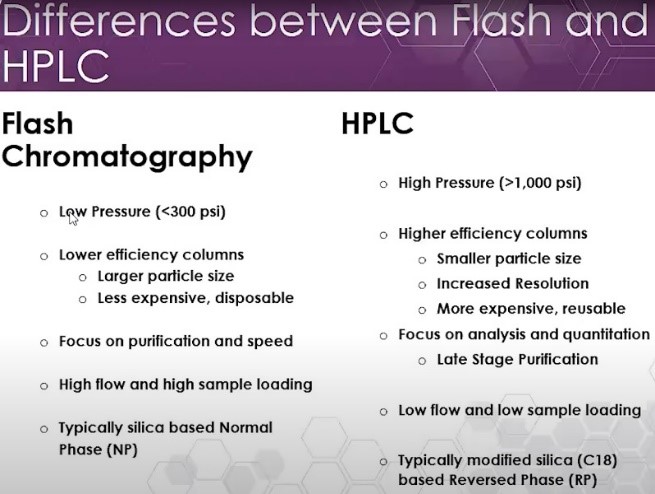
Flash chromatography, high-performance liquid chromatography (HPLC) and supercritical fluid chromatography (SFC) differ in the types of solvents used, the particle size of the stationary phase, and the pressure under which each operates. This affects the resolution and equipment needs for each technique.
HPLC and SFC send the mobile phase through the stationary phase under far greater pressure than in flash. This, and the use of smaller particles for the stationary phase in the column, gives HPLC and SFC a superior ability to distinguish between compounds and elute purer results.
Flash
| 20-60 µ
| >18 mL/min
| 10-50 PSI
| mg-g
|
Prep HPLC
| 5-20 µ
| 3-100 mL/min
| 200-2000 PSI
| 10-500 mg
|
Analytical HPLC
| 5 µ
| 1 mL/min
| 500-6000 PSI
| <5 mg
|



HPLC is divided into analytical and preparative forms. As you see in the chart, prep HPLC uses larger particles, meaning less surface area for the stationary phase; a higher flow rate; and, generally, less pressure than analytical HPLC. Flash and prep HPLC are used to purify large sample quantities; in analytical HPLC, the goal is to achieve the highest resolution possible in order to observe each unique compound in the sample.
Supercritical Fluid Chromatography
First, a very high-level description of “supercritical." The three states of matter are gas, liquid and solid. In the supercritical fluid region, matter under the influence of certain temperature and pressure levels becomes supercritical, where a difference between the gaseous and liquid states can no longer be observed.
In supercritical fluid chromatography (SFC), the mobile phase commonly used is supercritical carbon dioxide. Carbon dioxide is normally a gas (people and animals exhale it) and when it is under a certain pressure and cold enough, it forms dry ice. When the temperature and pressure reach a certain highpoint, the CO2 becomes supercritical.
Supercritical carbon dioxide is used in chromatography because it yields quick results and offers environmental advantages. It is stable, relatively inexpensive, non-flammable, non-toxic, and easy to remove from the sample. You can learn more about supercritical carbon dioxide here.
Because many compounds cannot be separated using supercritical CO2 alone, it usually is used in combination with another solvent, such as methanol, ethanol, and others like those discussed in the <link Thin Layer Chromatography section.
While the principles of SFC are much like those of HPLC, the supercritical fluid used as the mobile phase offers decreased viscosity, allowing SFC to be run at a higher flow rate than HPLC. Therefore, SFC is generally a three to four times faster process than HPLC. Other advantages of SFC over HPLC include its “green" features:
- Greatly reduces solvent consumption (up to eight times less organic solvent used)
- Less solvent waste
- Up to seven times lower energy consumption for solvent removal
- CO2-neutral: reuses CO2 captured from other processes or the atmosphere
- Environmentally friendly alternative to hazardous organic solvents
- CO2 is nontoxic and with appropriate co-solvents is safe to use with food and pharmaceutical products

 Teledyne ISCO's compact ACCQPrep® SFC system
Teledyne ISCO's compact ACCQPrep® SFC system
Be sure to check out the other blogs in this 4 part series:
